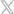EOlife: The First Smart Device that Measures the Quality of Manual Ventilation Gets FDA Clearance

With the recent FDA clearance of EOlife® by Archeon Medical, high-performance ventilation will become a standard of care. This approval marks a significant milestone for Archeon Medical on its mission to promulgate the practice of high-performance ventilation. (1) A 2019 study showed that EOlife improves the performance of manual ventilation by over 70 %. Which in turn can triple the chance of patient survival.
EOlife Device and What it Offers:
EOlife is an easy-to-use medical device that helps clinicians efficiently ventilate patients in cardiac arrest. EOlife has an AI system that measures ventilation parameters and provides first responders with real-time feedback on the quality of manual ventilation provided to the patient. This portable device is designed for extreme environments. EOlife is drop/vibration resistant, water resistant, and can withstand temperatures between -4°F and 122°F. Other features include a 5-hour run time and its numerous live statistics that provide first responders with crucial information when it matters most.
What High-performance Ventilation Encompasses:
- Providing adequate volume while minimizing the risk of gastric inflation
- Avoiding excessive gas leakage which can result in inadequate ventilation of the patient’s lungs
- Avoiding hyperventilation
EOlife Allows Users to put the “P” Back in CPR.
- Choose the appropriate mask size
- Position and seal the airway mask more effectively to prevent air leaks
- Correctly use the jaw-thrust maneuver to avoid insufflation of air into the stomach
- Squeeze the bag and adapt the delivered volume and rate to the patient to avoid hyperventilation
Archeon Medical also offers EOlife X®, a CPR manual ventilation training device. Learn more
For a demonstration or more information about the EOlife from Archeon, contact your local MED Alliance Group sales representative, call 888-891-1200, or email us.
MED Alliance Group is a medical device distributor that has been meeting the needs of our clinical customers and manufacturing partners since 1998. We specialize in the sales, marketing, importation, logistics, and distribution of innovative, high-quality, and cost-effective products found in anesthesia and respiratory, blood and transfusion therapy, EMS and emergency room, interventional radiology, and cath lab, iv and vascular, NICU/PICU, and pharmacy.
Please follow us on LinkedIn, Facebook , and Twitter for MED Alliance product updates.
Reference
(1) Khoury A, De Luca A, Sall FS, Pazart L, Cappelier G. Ventilation feedback device for manual ventilation in simulated respiratory arrest: a crossover manikin study. Scand J Trauma Resusc Emerg Med. 2019 Oct 22;27(1):93