Improving OHCA Survival with Favorable Neurological Outcomes: Good BVM Ventilation Matters
Out-of-Hospital Cardiac Arrest (OHCA) Outcomes
The American Heart Association estimates more than 350,000 out-of-hospital cardiac arrests (OHCA) occur in the U.S. each year, with a survival rate of only 10%. Unfortunately, up to half of these survivors experience cognitive problems due to a lack of oxygen during resuscitation.1 (AHA)
Beating the odds and surviving cardiac arrest is certainly worth celebrating, however, quality of life after survival is also a concern.
Emergency responders know the importance of oxygenation during resuscitation and that bystander chest compressions performed before their arrival have not provided sufficient oxygenation. First responders will immediately deliver oxygen, typically with a bag-valve mask, to avoid hypoxic-ischemic brain injury.
However, studies indicate many experienced medical professionals struggle to perform adequate BVM ventilation. Inadequate ventilation during cardiac arrest management can lead to severe neurological complications affecting quality of life.
Proper Ventilation Leads to Fivefold Increase in Survival with Favorable Neurological Outcomes
A 2023 multicenter study led by Dr. Ahamed Idris with the University of Texas Southwestern Medical Center sought to determine the incidence of lung inflation with BVM ventilation during 30:2 CPR and the association of ventilation on patient outcomes after OHCA.
Researchers reviewed thoracic bioimpedance waveforms in 1976 OHCA patients and found that most pauses did not have detectable ventilation (≥250 mL of tidal volume). Sixty percent of patients had ventilation waveforms in less than 50% of pauses and 40% had waveforms in more than 50% of the pauses.
The study concluded that lung inflation occurs infrequently with BVM ventilation during 30:2 CPR for OHCAs. However, when proper ventilation was provided (adequate tidal volume) there was a fivefold increase in survival with favorable neurological outcomes (10.6% versus 2.4%).
The authors of the Idris study state that providing adequate ventilation through BVM is a skill that must be practiced to maintain proficiency.
Click here to view the study in full.
Improving Manual Ventilation Skills
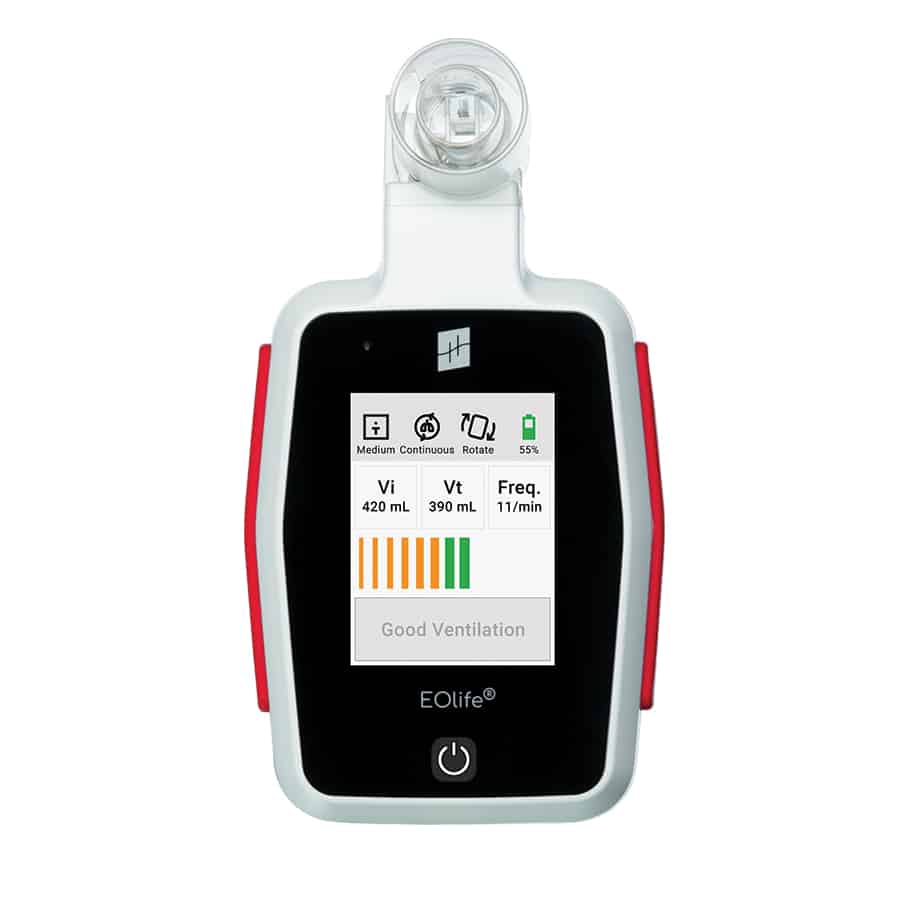
The EOlife and EOlife X from Archeon Medical are ventilation feedback devices that provide real-time measurements to enhance ventilation precision.
Perform high-performance ventilation during training and in the field, regardless of experience. The devices help users ensure adequate volume while minimizing gastric inflation risk, avoid excessive gas leakage and hyperventilation.
Both devices adapt to any bag, mask and tracheal tube to measure and give real-time feedback on:
- Insufflated volume
- Tidal volume
- Ventilation rate
- Gas leakage
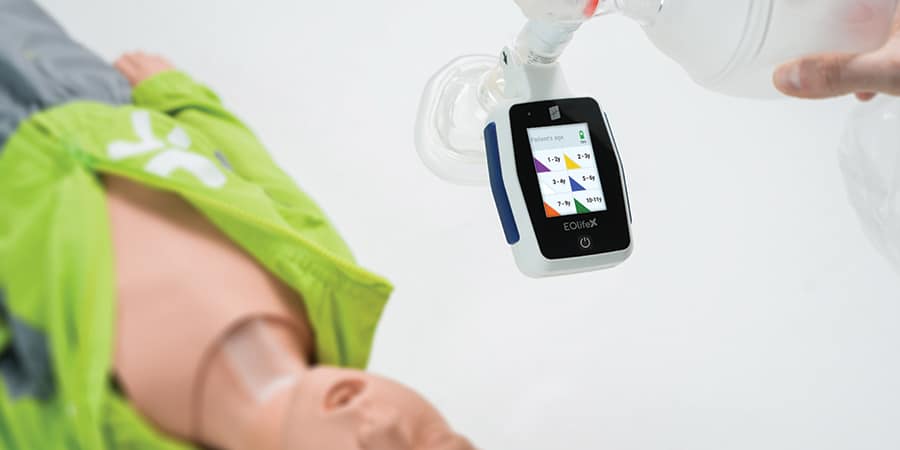
EOlife X: The Ultimate Training Tool for High-Performance Ventilation
EOlife: The Ultimate Feedback Device for High-Performance Ventilation
EOlife X teaches first responders about proper airway management and good ventilation regardless of the user’s level of experience and patient profile. With adult and pediatric settings, the device provides visual feedback on the quality of ventilation with two training modes (blind or monitored) that record ventilation parameters. Data can be downloaded via Bluetooth to the EOlife Connect app which offers detailed analysis and allows users to track their skills progress over time.
EOlife is an FDA-cleared, reliable and intuitive medical device that measures ventilation parameters and can provide users with real-time feedback on the quality of manual ventilation to the patient. EOlife offers two different user interfaces depending on the type of user (ALS or BLS). By providing visual feedback, the device increases the quality of manual ventilation by 70%, reduces the risk of hyperventilation tenfold and can triple the chance of patient survival.
For more information and to request a demonstration of EOlife X and EOlife, call 888-891-1200 or email us to be connected to your local sales representative.
MED Alliance Group is a medical device distributor that has been meeting the needs of our clinical customers and manufacturing partners since 1998. We specialize in the sales, marketing, importation, logistics and distribution of innovative, high-quality and cost-effective products found in anesthesia and respiratory, blood and transfusion therapy, EMS and emergency room, interventional radiology and cath lab, iv and vascular, as well as NICU and PICU.
Please follow us on LinkedIn, Facebook and Twitter for MED Alliance product updates.