Make False Positive Blood Culture Results a Thing of the Past
Blood cultures are important diagnostic tools for identifying the pathogen(s) responsible for a patient’s infection. This is especially true of patients with suspected sepsis or septic shock and for patients with suspected infective endocarditis.¹
Unfortunately, blood culture contamination is a significant problem that can lead to false positive results, misdiagnosis, and costly, delayed, or inappropriate treatments including antibiotic misuse. According to the American Hospital Association, over 1.2 million contaminated blood cultures occur in the U.S. annually.²
Keeping blood culture contamination rates low should be at the forefront of every institution. In fact, there is a national movement to lower the benchmark for blood culture contamination from 3% to 1%. The College of American Pathologists recommends that the laboratory director should regularly review blood culture contamination rates. Regularly reviewing contamination rates and providing feedback to units and persons drawing blood cultures is one method that has been shown to reduce contamination rates.³ When best practices are followed and the right tools are used, a target contamination rate of 1% or lower is achievable.
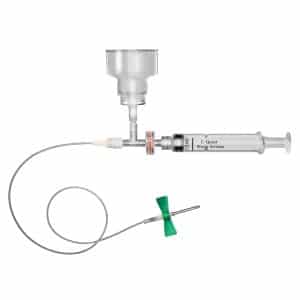
Sequestration Syringe™: The Right Tool for Accurate Blood Culture Collection
The Sequestration Syringe Blood Culture Diversion Collection Device with proprietary initial specimen diversion technology is setting new standards in blood culture collection. Available at half the cost of competitive devices, the Sequestration Syringe is an easy-to-use way to achieve accurate blood culture results and reduce false positive rates.
The Sequestration Syringe features a proprietary Unidirectional Check Valve that automatically diverts and sequesters the initial 1.5mL of blood. The valve assures that blood can only flow in one direction, to prohibit sequestered blood from contaminating the culture. Unlike unfamiliar and costly diversion alternatives, the Sequestration Syringe features a familiar, clinician-friendly syringe design that requires virtually no training. This results in greater efficiency and cost savings while promoting standardization for rapid, accurate blood culture collection procedures throughout the hospital.
Each Sequestration Syringe is packaged with a complete C-Quest Blood Culture Collection Kit, including a sterile initial specimen diversion tube. The kit contains everything needed for pre-collection, collection, and post-collection applications – all in one place, saving clinicians time and enhancing process improvement and compliance.
Features and Benefits
• Proprietary “T” Valve and Unidirectional Check Valve can be used for either venipuncture or IV draw
• Unidirectional Check Valve diverts and sequesters initial 1.5mL ash of blood
• Improves clinical outcomes
• Enhances proper patient diagnosis and treatment
• Reduces antibiotic use and length of hospital stay
• Clinician-friendly, intuitive design is easy to use
• Requires minimal in-service training
• FDA 510(k) Clearance
References:
(2) “The Impact and Prevention of False Positive Clabsis: AHA.” American Hospital Association, www.aha.org/education-events/impact-and-prevention-false-positive-clabsis#:~:text=If%20a%20patient%20has%20a,unnecessary%20CLABSI%20reporting%20is%20significant. Accessed 29 July 2024.
For more information about the Sequestration Syringe or to request a demonstration, call 888-891-1200 or email us to be connected to a local representative.
MED Alliance Group is a medical device distributor that has been meeting the needs of our clinical customers and manufacturing partners since 1998. We specialize in the sales, marketing, importation, logistics and distribution of innovative, high-quality and cost-effective products found in anesthesia and respiratory, blood and transfusion therapy, EMS and emergency room, interventional radiology and cath lab, iv and vascular, NICU/PICU and pharmacy.
Please follow us on LinkedIn, Facebook and Twitter for MED Alliance product updates.