MED Alliance International Welcomes IMBiotechnologies Ltd

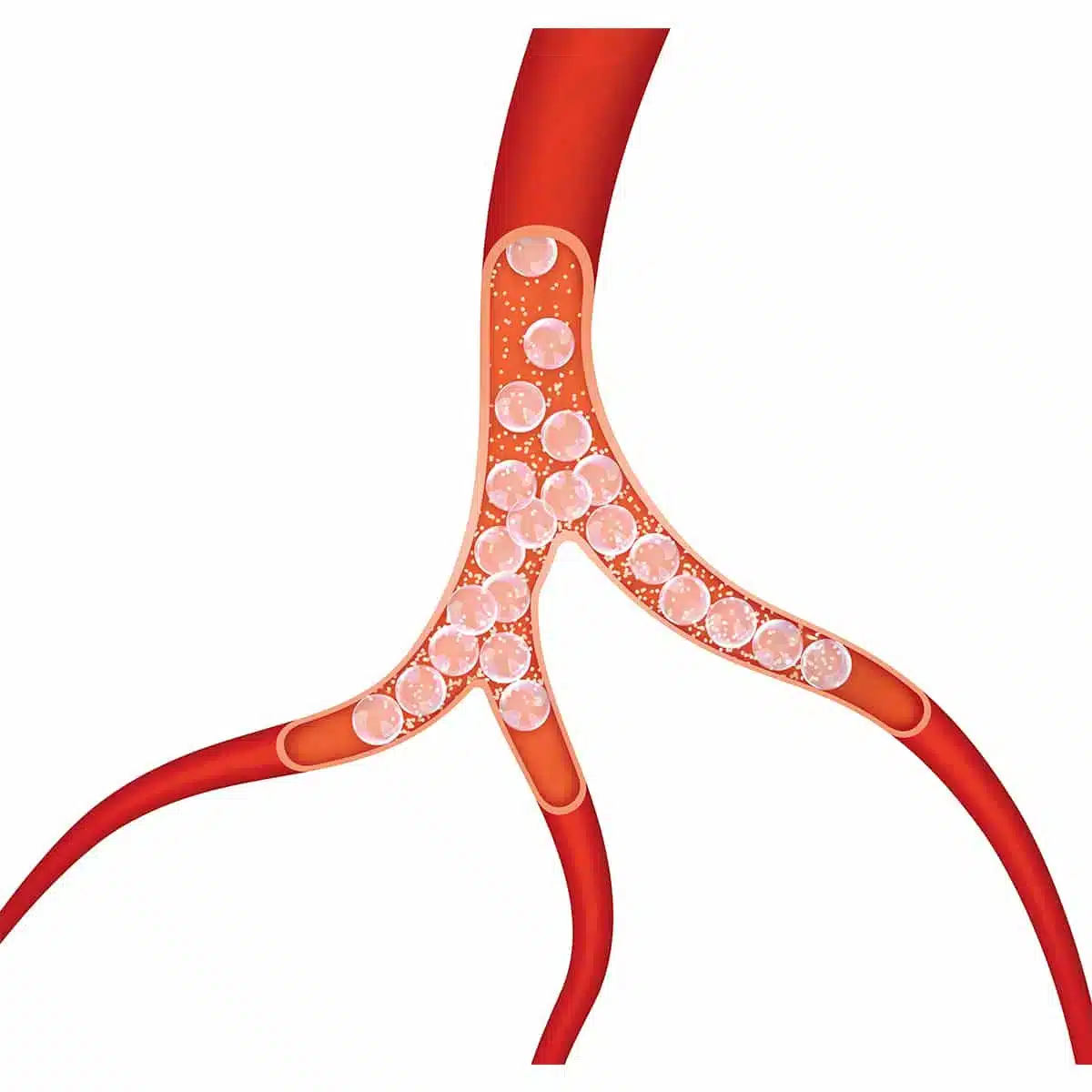
MED Alliance International, a division of MED Alliance Group, LLC, is excited to partner with IMBiotechnologies Ltd (IMB), the manufacturer of Ekobi™ Embolization Microspheres (Ekobi) used in therapeutic embolization procedures. Ekobi is a the only embolic agent that is biodegradable and detectable by ultrasound.
IMB, a Canadian-based company, is partnering with MED Alliance to provide logistical and operational support to provide Ekobi to U.S. and Canadian clinical customers. IMB’s mission is to create and commercialize innovative products that treat human disease and improve quality of life. Ekobi is approved in Canada for the treatment of hypervascularized tumors, uterine fibroids, and enlarged prostates due to benign prostatic hyperplasia. Ekobi is cleared for the treatment of unresectable/inoperable hypervascularized tumors in the U.S.
“MED Alliance International’s strong relationships with U.S. hospitals can benefit companies like IMB who are working toward expanding their market share in the U.S. and Canada,” said MED Alliance Vice President of Operations and Finance Lindsey Allende. “We take the guesswork out of the equation, eliminate barriers and provide channels to help our partners grow.”
“MED Alliance provides key logistical support in supplying Ekobi to IMB’s customers in a timely manner. This partnership allows IMB to meet market demand for this innovative product. Ekobi delivers a therapeutic benefit then biodegrades; it is not a permanent implant,” said IMB CEO Michael Stewart. “Ekobi is directly imaged using standard ultrasound, providing a distinct advantage in embolization procedures.”
Learn more about IMBiotechnologies and Ekobi Microspheres.