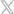RadTag®: An innovative way to confirm that blood products are irradiated
Every two seconds someone in the U.S. needs blood and or platelets, and nearly 16 million blood components are transfused each year.¹ Donated blood can be the key to saving lives. The devices used to collect, process, store, transport, and deliver blood and blood products are instrumental in protecting this life-saving donation and ensuring it is safe to transfuse.
Irradiation is an important process that prevents a rare but serious disease called transfusion-associated graft-versus-host disease (TA-GVHD) that can occur during blood transfusions. TA-GVHD occurs in 0.1–1.0% of susceptible recipients.² Although TA-GVHD is rare, it is an extremely dangerous disease as the mortality rate is 87–100% because bone marrow failure is almost universal in patients with TA-GVHD.³
Put Patient Safety First with RadTag Precision
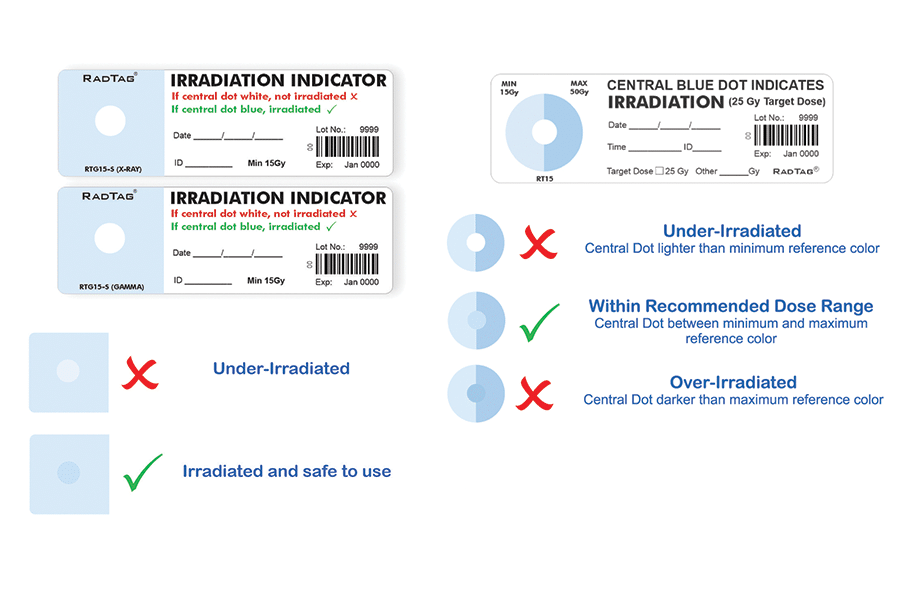
RadTag® indicators offer the best final validation that irradiated blood is safe to transfuse. Users can visually validate if blood received the required minimum dose or exceeded the maximum allowable dose as recommended by FDA and AABB guidelines. No other indicator accurately provides this functionality.
RadTag indicators utilize a flexible adhesive label that is easy to apply to blood bags and syringes. Users can quickly and easily visually interpret the unique color-changing material that gives a clear indication upon exposure to ionizing radiation.
The sensitive portion of the indicator is white initially and changes to a blue color upon exposure to ionizing radiation; the shade of blue is an approximation of the amount of radiation dose delivered. The color change is permanent and immediate and does not require any further processing or development.
RadTag Offers Multiple Indicator Options:
- Minimum/Maximum Dose Verification for Irradiation
- Minimum Dose Verification for Gamma Irradiation
- Minimum Dose Verification for X-ray Irradiation
References:
1 “US Blood Supply Facts.” Facts About Blood Supply In The U.S. | Red Cross Blood Services, www.redcrossblood.org Accessed 5 Sept. 2024.
2 & 3 Patel, Ketan K, et al. “Transfusion Associated Graft versus Host Disease Following Whole Blood Transfusion from an Unrelated Donor in an Immunocompetent Patient.” Indian Journal of Hematology & Blood Transfusion : An Official Journal of Indian Society of Hematology and Blood Transfusion, U.S. National Library of Medicine, Sept. 2010, www.ncbi.nlm.nih.gov
For samples, a demonstration or more information about the RadTag, contact your local MED Alliance Group sales representative, call 888-891-1200 or email us.
MED Alliance Group is a medical device distributor that has been meeting the needs of our clinical customers and manufacturing partners since 1998. We specialize in the sales, marketing, importation, logistics and distribution of innovative, high-quality and cost-effective products found in anesthesia and respiratory, blood and transfusion therapy, EMS and emergency room, interventional radiology and cath lab, iv and vascular, NICU/PICU and pharmacy.
Please follow us on LinkedIn, Facebook and Twitter for MED Alliance product updates.