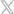Reducing IV Dislodgements, Reconnects and IV Site Complications
IV Dislodgements Pose A Safety Risk to Patients
Despite the use of catheter securement and dressings, the dislodgement rates for IVs still range from 1.8 to 24%. This poses a significant risk to patients as it can lead to complications and the need for IV reconnects, which require additional hospital resources and staff time.
Catheter movement puts patients at risk of conditions such as phlebitis, infiltration, extravasation, IV infection, and hematoma. The repercussions of IV dislodgement, including delays in patient treatment and procedures, can also compromise patient safety.
While catheter securement has made significant advancements, there is still more that can be done to prevent IV dislodgements.
Solution: The Orchid Safety Release Valve
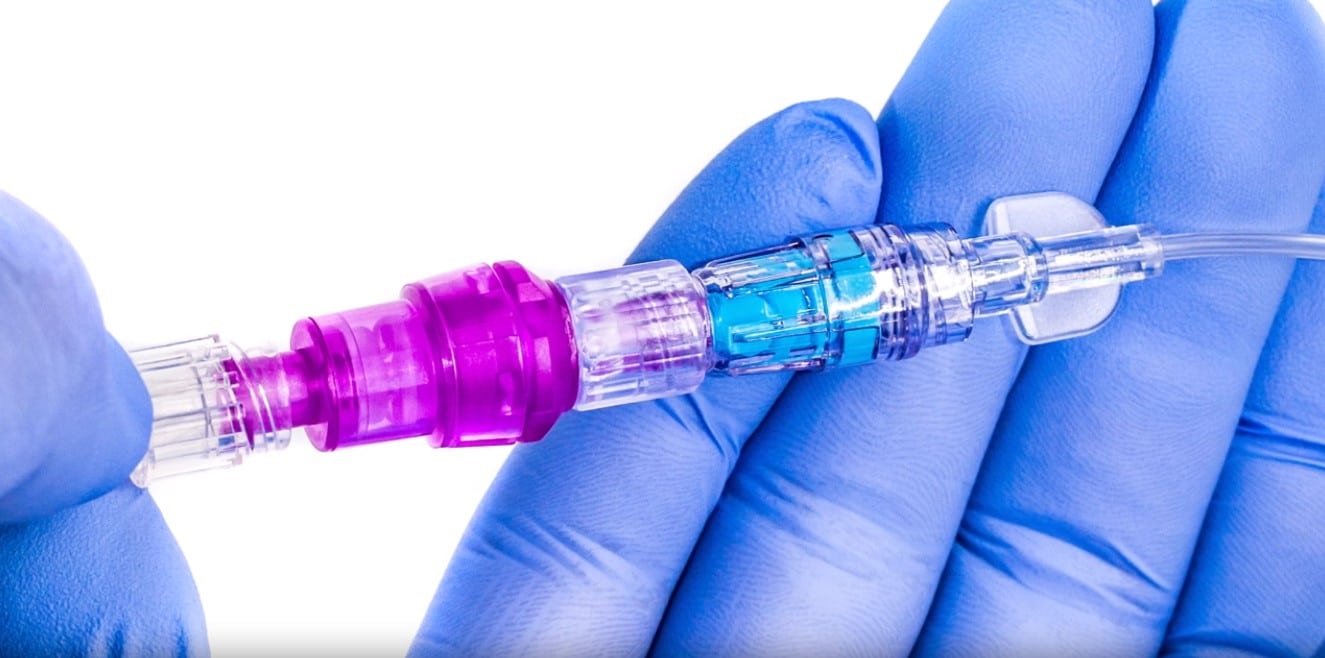
The Orchid SRV™ (Safety Release Valve) from Linear Health Sciences is a sterile, single-patient-use connector for needle-free access, placed between the existing IV extension set and general IV tubing connection intended to be used for delivery of fluid to and from an IV catheter. The device can be used with all catheters during direct injection, intermittent infusion, and continuous infusion on patients aged two weeks and up.
The Orchid SRV is proven to prevent IV dislodgement by up to 91.9%
When tension across the device exceeds a predetermined threshold, the device separates into two halves, activating valves that create sterile seals for the fluid pathway to retain IV site integrity. When the flow stops, a downstream occlusion alarm will alert a clinician who will remove and replace the separated Orchid SRV halves with a new, sterile Orchid SRV.
Every Line. Every Time.
Break away from the expense and pain of IV dislodgements. The Orchid SRV helps to:
- Retain continuity of care
- Avoid unnecessary IV restarts
- Decrease exposure to Sharps injuries
- Achieve greater patient safety and satisfaction
- Focus on value-added nursing time
- Save valuable hospital funds
Click here to learn more.
To learn more about the Orchid Safety Release Valve or to request an evaluation, call 888-891-1200 or email us to be connected to a local representative.
MED Alliance Group is a medical device distributor that has been meeting the needs of our clinical customers and manufacturing partners since 1998. We specialize in the sales, marketing, importation, logistics and distribution of innovative, high-quality and cost-effective products found in anesthesia and respiratory, blood and transfusion therapy, EMS and emergency room, interventional radiology and cath lab, iv and vascular, NICU/PICU and pharmacy.
Please follow us on LinkedIn, Facebook and Twitter for MED Alliance product updates.