Whitepaper: The Impact and Safety Risks of Accidental IV Dislodgement
This is a summary of a Whitepaper from Nancy Moureau, PhD, RN, CRNI, CPUI, VA-BC. To download the Whitepaper in its entirety with references, click here.
Accidental Dislodgement of Vascular Access Devices
Accidental dislodgement — a major contributor to catheter failure and ensuing treatment delays, is prevalent among adult and pediatric populations, yet it is often underrecognized as a serious medical concern by clinical staff because it happens practically every day. More than 5 million catheters are dislodged annually underscoring the need for viable solutions to address this ongoing issue.
In an ECRI Polyurethane Medical Device Material Safety Report published in 2021, peripheral arterial catheters, peripherally inserted central catheters (PICC), and central venous catheters (CVC) were cited as having the highest rates of complete dislodgement.
In the US, costs associated with tubing disconnection, catheter failure, and restarts are estimated at more than $7 billion; therefore, accidental dislodgement represents not only a patient safety issue but also a significant economic burden.
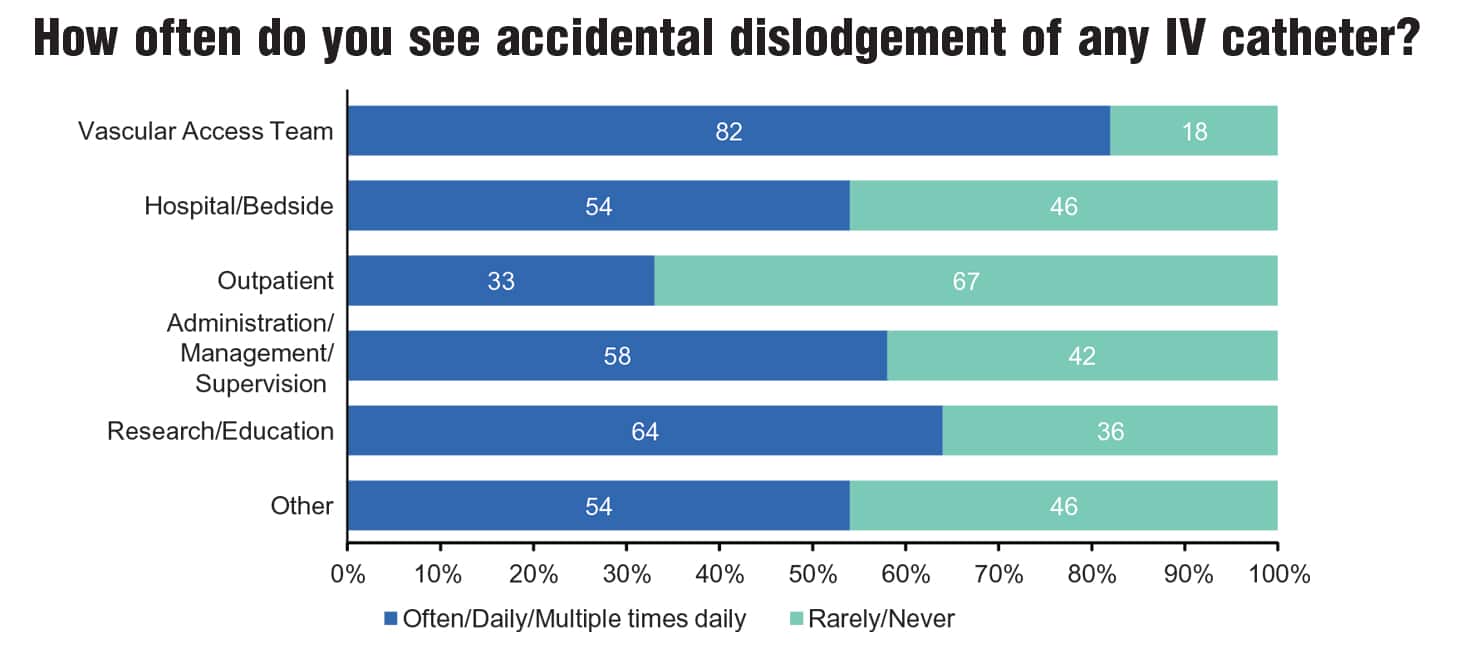
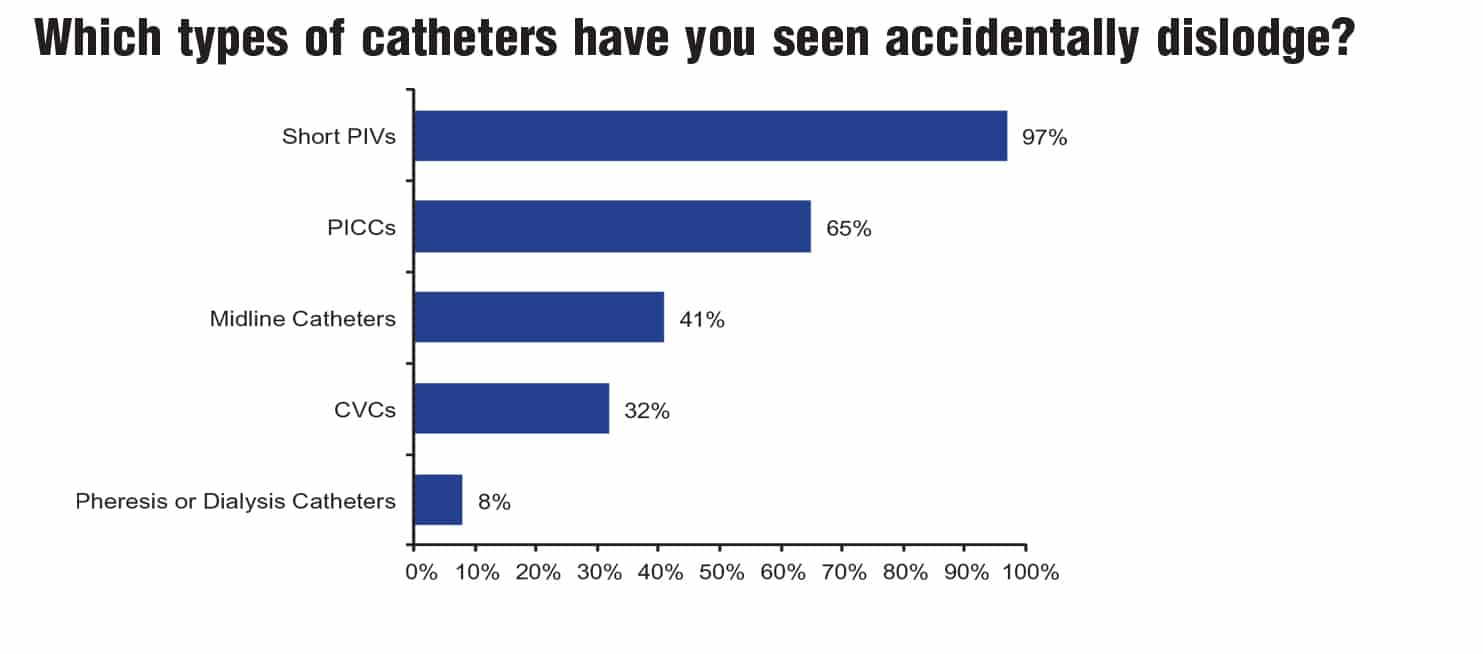
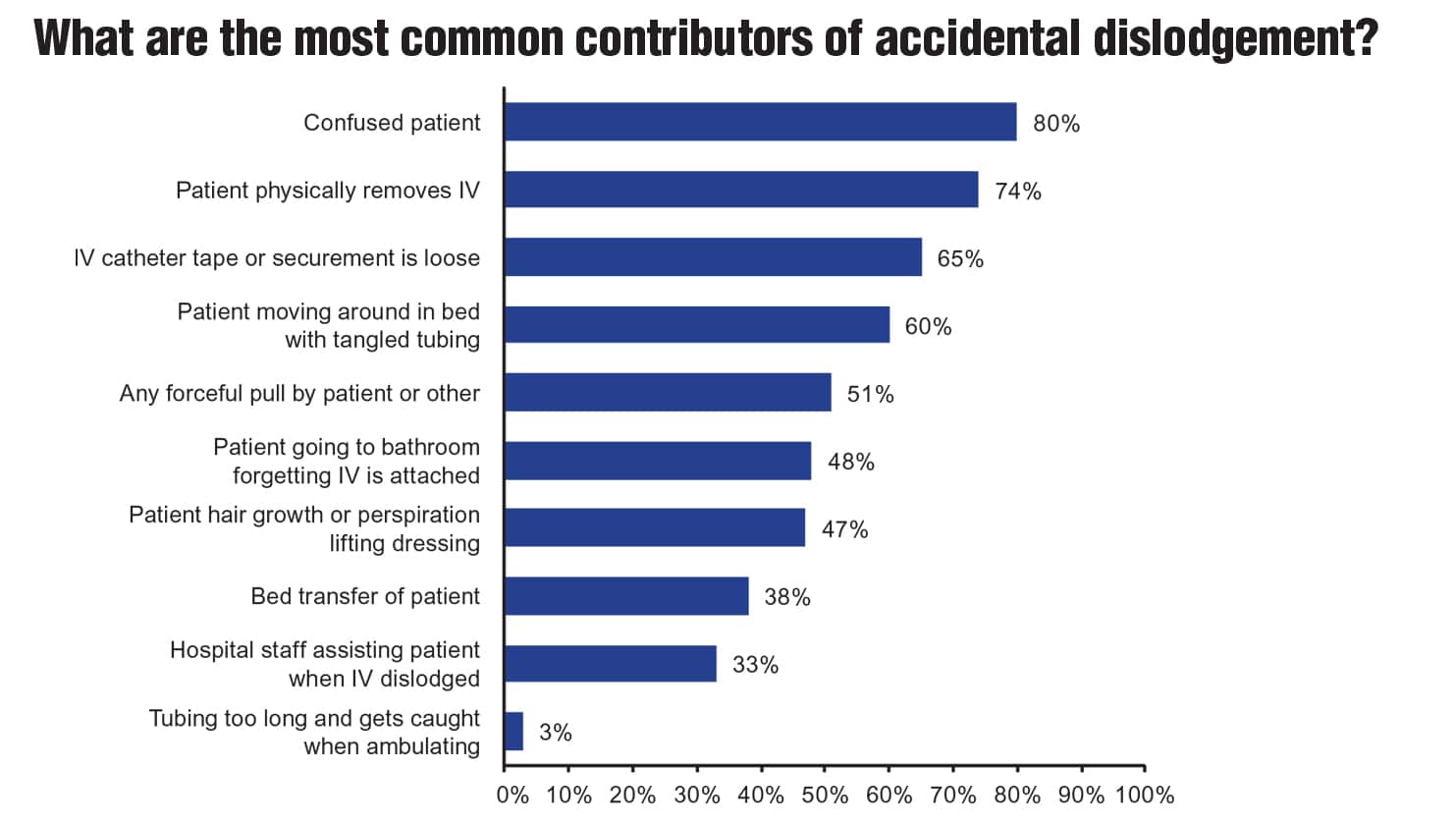
A research survey conducted by Moureau examined clinician perceptions and experiences related to accidental dislodgement of IV devices. Sixty-eight percent of the 1,561 respondents reported observing accidental dislodgement daily, often multiple times in one day (Figure 1A). Consistent with findings from other studies, 96.5% of respondents reported that these occurrences happened primarily with peripheral IV catheters (Figure 1B). Furthermore, respondents indicated that patient confusion (80%), physical removal of the catheter by the patient (74%), and loose IV catheter or securement tape (65%) were the top three factors contributing to accidental dislodgement of IV devices (Figure 1C).
Unsurprisingly, most of the clinician participants agreed that accidental dislodgement represents a serious safety concern and that it was inadequately addressed by their care facility. These findings highlight the need for effective and easy-to-integrate solutions to mitigate the risk of accidental dislodgement.
Solution: The Orchid Safety Release Valve
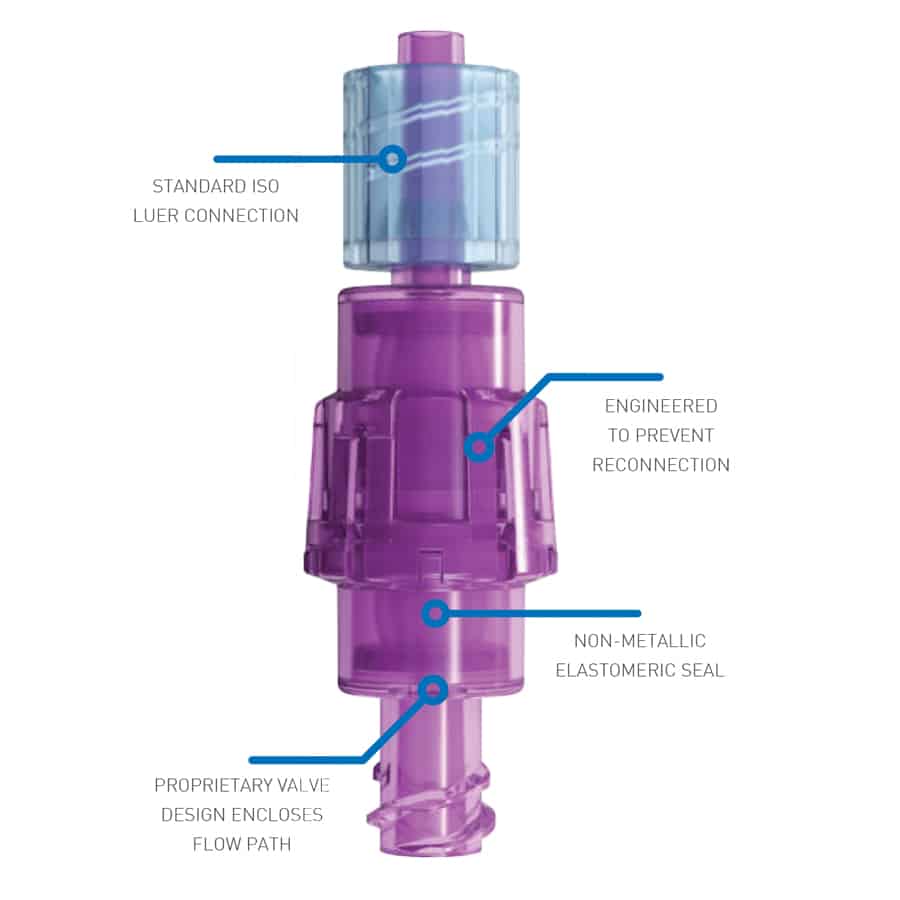
The Orchid SRV™ (Safety Release Valve) from Linear Health Sciences is a sterile, single-patient-use connector for needle-free access. It is designed to protect against accidental or unwanted IV dislodgement.
Mechanical catheter dislodgement occurs when a pull force between 1 to 8 lbs is applied. Although catheter and securement tape are useful for increasing the stability of the IV device and reducing vein irritation while it is inserted, securement alone cannot withstand the force applied during pulling, jerking, and other forceful movements.
The Orchid SRV is placed between the existing IV extension set and the general IV tubing connection intended for use in the delivery of fluid to and from a PIV catheter. The safety release device is installed within the tubing of IV or intra-arterial administration sets for continuous or intermittent infusions. When the tension force reaches 3.25 pounds of force (lbf), the valve separates.
Activation of the separation mechanism creates an automatic sterile seal for the fluid pathway. The seal is designed to stop the infusion and preserve the IV catheter for continued use. Once separated, the two parts of the SRV remain attached to the tubing end and the needleless connector catheter end. When the seal is activated and infusion flow ceases, an occlusion alarm alerts the clinician to check and replace the SRV device. Then the clinician reconnects a new SRV onto IV tubing and catheter access by simply removing the separated halves and replacing them with the new, prepackaged, sterile valve using an aseptic technique.
The feature is particularly useful when clinical staff need to disconnect the IV tubing to allow patients to go to the bathroom or change their clothes without the concern over introducing microbes into the IV line.
Stress testing in simulated clinical conditions revealed the Orchid SRV prevented IV dislodgements by 91.9% across all testing scenarios and experimental groups.
Accidental dislodgement is associated with significant healthcare costs. Available estimates suggest that the average cost to replace a dislodged IV catheter in the US is $50. The cost of replacing the catheter alone is extrapolated to be $2500 per day, or up to $912,500 annually for a typical 200-bed facility. Therefore, preventing even a fraction of accidental dislodgements would result in significant healthcare savings.
CONCLUSION
Accidental dislodgement is a serious, yet largely preventable complication associated with IV catheters. Catheter failure, treatment delays, injury, and the need for IV device replacements can result in extended hospital stays and significantly raise healthcare costs. Clinicians agree that the dislodgement of IV devices is a significant medical event that occurs daily but is not adequately addressed in healthcare facilities. Orchid SRV is a cost-effective, easy-to-use device that separates into two aseptically sealed compartments when activated by pull forces ≥ 3 lbs. It is compatible with existing IV sets and standard securement products. Performance testing leading to the approval of SRV demonstrates that it can prevent approximately 90% of accidental dislodgments. This novel device represents an effective solution for addressing the clinical challenge of accidental IV device dislodgements in acute and at-home settings.
To review the Whitepaper in its entirety with references, click here.
Every Line. Every Time.*
Break away from the expense and pain of IV dislodgements.
The Orchid SRV helps to:
- Retain continuity of care
- Avoid unnecessary IV restarts
- Decrease exposure to Sharps injuries
- Achieve greater patient safety and satisfaction
- Focus on value-added nursing time
- Save valuable hospital funds
Click here to learn more.
*Refer to IFU for all indications
To learn more about the Orchid Safety Release Valve or to request an evaluation, call 888-891-1200 or email us to be connected to a local representative.
MED Alliance Group is a medical device distributor that has been dedicated to meeting the needs of our clinical customers and manufacturing partners since 1998. We specialize in the sales, marketing, importation, logistics and distribution of innovative, high-quality and cost-effective products found in anesthesia and respiratory, blood and transfusion therapy, EMS and emergency room, interventional radiology and cath lab, iv and vascular, NICU/PICU and pharmacy.
Please follow us on LinkedIn, Facebook and Twitter for MED Alliance product updates.